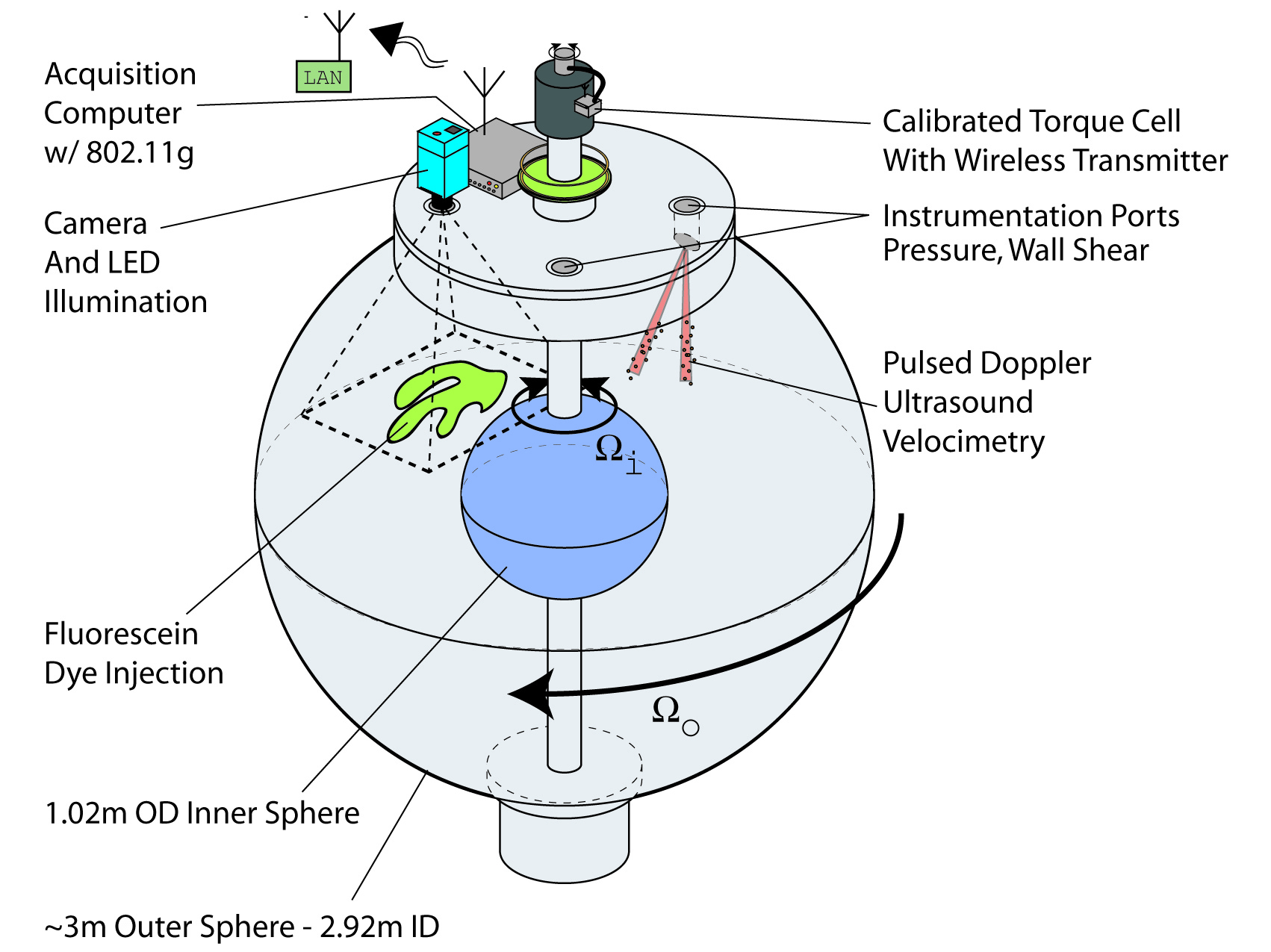
Inner And Outer Sphere Complex
C) First consider electron transfer between the ruthenium complex and the cobalt complexes. Are these reactions more likely to occur via an inner sphere or an outer sphere mechanism? D) The researchers measured rates of electron transfer between the ruthenium and each of the cobalt complexes. The β-ammonioethyl complex of platinum (IV), KPt(CH2CH2NH3)Cl5, undergoes two types of transformations in aqueous chloride solutions,i.e., inner- and outer-sphere reductive elimination, depending on the pH of the medium. In acidic solutions outer-sphere decomposition, which consists of an SN2 attack by the chloride ion of the α-carbon atom of the complex anion trans-Pt(CH2CH2NH3)Cl4(H2O.
Difference between inner and outer sphereFirstly, electron transfer reactions between metal complexes can be divided into two categories: outer sphere and inner sphere. In the former, the two complexes come together and an electron is transferred without any bond being formed between the complexes.
In the latter, the two complexes form a definite intermediate where at least one ligand is shared by both metal ions.In inner sphere reactions, of course, a ligand must be substituted on one metal ion by the ligand on the other metal ion that forms the bridge. Therefore if electron transfer is observed to occur faster than either complex undergoes ligand exchange reactions, this is good evidence that the mechanism for electron transfer is outer sphere. If neither complex has any ligands capable of bridging (that is, with extra lone pairs), the mechanism must be outer sphere.FRANK CONDON PRINCIPLEThe free energy of activation D G ‡ for (I) is 33 kJ mol –1, and the second order rate constant is 3 L mol –1 s –1 at 25?C.
It is worth considering why there is a barrier, even though quite small, to a reaction in which there is no net chemical change, and for which therefore D G must be zero.Basically, what is happening is that an electron from the Fe(II) t 2g orbital is transferred to the Fe(III) t 2g orbital. For this to happen without energy input, the energies of the two orbitals must be the same (Franck–Condon principle). But clearly, they will not be; Fe(H 2 O) 6 2+ is different from Fe. (H 2 O) 6 3+.
The Swindlers is a 2017 South Korean crime action film directed by Jang Chang-won. The film stars Hyun Bin, Yoo Ji-tae, Bae Seong-woo, Park Sung-woong, Nana and Ahn Se-ha. Directed by Chang Won Jang. With Hyun Bin, Ji-Tae Yoo, Sung-Woo Bae, Sung-woong Park. Unlikely allies from different sides of the law must work together to achieve a common goal - trapping the world's most legendary con man. The swindlers korean movie.
Its metal–ligand bonds will be longer because the Fe 2+ –O electrostatic interaction will be less than that of Fe 3+ –O. If electron transfer were able to take place without energy input, we would end up with Fe(H 2 O) 6 3+ with bond lengths typical of Fe(H 2 O) 6 2+ and Fe. (H 2 O) 6 2+ with bond lengths typical of Fe. (H 2 O) 6 3+. Both could then relax with the release of energy, violating the first law of thermodynamics.What has to happen to allow electron transfer is that the Fe(II) complex has to be in an excited vibrational state. At some time, its Fe–O bonds will be shortened somewhat, compared with the ground state.
This will raise the energy of the t 2g orbitals as there will then be greater repulsion of electrons in these orbitals by the water lone pairs. The Fe(III) complex has also to be in an excited vibrational state so that at some times, its bonds will be lengthened compared with the ground state, and the energy of the t 2g orbitals will be lower than in the ground state.
This is the origin of some of the activation energy for electron transfer.Contributions to energy of activationThere are other contributions to the energy of activation. For example, both the ions in our Fe(II)/Fe(III) example are positively charged, so this would present a barrier to them coming together close enough to allow electron transfer to occur. There is also energy required to adjust the ‘second coordination sphere’ of solvent ( i.e.
The solvent which is strongly hydrogen-bonded to the coordinated waters) around the participating ions, from the ground state arrangement to the transition state arrangement. This contribution to the free energy of activation is called the ‘solvent reorganisation term’, and can be quite large.D G ‡ (total) = D G ‡(bond length changes) + D G ‡(solvent reorg.) + D G ‡(Coulombic)The bigger the difference in metal–ligand bond lengths between oxidant and reductant, the slower will be the electron transfer reaction, because the free energy of activation will be higher. In the Fe(II)/Fe(III) case mentioned above, the barrier is quite small because both Fe(II) and Fe(III) give high spin complexes with water. Fe(II) is therefore t 2g 4 e g 2 and Fe(III) is t 2g 3 e g 2. The difference in bond lengths is comparatively small and is mostly due to the difference in oxidation state.An example where there is a much bigger difference concerns reaction ( III) below.Co(NH 3 ) 6 3+ + Co. (NH 3 ) 6 2+ ® Co(NH 3 ) 6 2+ + Co.
(NH 3 ) 6 3+ ( III)Co–N 1.936(15) A 2.114(9) AThe second order rate constant for this reaction is about 10 –6 dm 3 mol –1 s –1 (i.e. Very slow) and D G ‡ is over 100 kJ mol –1. This is because the Co(III) complex is low spin, and therefore t 2g 6 e g 0. However, the Co(II) complex is high spin, t 2g 5 e g 2.
The presence of two extra electrons in the e g orbitals, repelling the ligands, means that the Co–N bonds in the Co(II) complex are much longer than in the Co(III) complex, so the two complexes must both be highly vibrationally activated before electron transfer can occur.Marcus theoryThe ideas shown above were expressed quantitatively by R.A. Marcus (Nobel Prize for Chemistry 1992).
 The Way of the Samurai series is widely renowned for allowing players to make far-reaching decisions that can radically alter the course of the story and this, the fourth entry in this thrilling series and previously exclusive to the PS3, is no exception!
The Way of the Samurai series is widely renowned for allowing players to make far-reaching decisions that can radically alter the course of the story and this, the fourth entry in this thrilling series and previously exclusive to the PS3, is no exception!
The uranyl( VI) complex UO 2Cl(L) of the redox-active, acyclic diimino-dipyrrin anion, L − is reported and its reaction with inner- and outer-sphere reductants studied. Voltammetric, EPR-spectroscopic and X-ray crystallographic studies show that chemical reduction by the outer-sphere reagent CoCp 2 initially reduces the ligand to a dipyrrin radical, and imply that a second equivalent of CoCp 2 reduces the U( VI) centre to form U( V). Cyclic voltammetry indicates that further outer-sphere reduction to form the putative U( IV) trianion only occurs at strongly cathodic potentials. The initial reduction of the dipyrrin ligand is supported by emission spectra, X-ray crystallography, and DFT; the latter also shows that these outer-sphere reactions are exergonic and proceed through sequential, one-electron steps. Reduction by the inner-sphere reductant TiCp 2Cl 2 is also likely to result in ligand reduction in the first instance but, in contrast to the outer-sphere case, reduction of the uranium centre becomes much more favoured, allowing the formation of a crystallographically characterised, doubly-titanated U( IV) complex.
In the case of inner-sphere reduction only, ligand-to-metal electron-transfer is thermodynamically driven by coordination of Lewis-acidic Ti( IV) to the uranyl oxo, and is energetically preferable over the disproportionation of U( V). Overall, the involvement of the redox-active dipyrrin ligand in the reduction chemistry of UO 2Cl(L) is inherent to both inner- and outer-sphere reduction mechanisms, providing a new route to accessing a variety of U( VI), U( V), and U( IV) complexes.